

 />
/>

Mequindox
Cas No:60875-16-3
Molecular formula:C11H10N2O3
Molecular weight:218.21
Annual production:
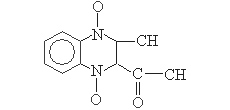
Structural formula:
Veterinary drug GMP certificated company approved by Ministry of Agriculture
Manual of Veterinary Mequindox
[Veterinary Drug Name]
Common name: Mequindox
Product Name: Mequindox
Product name: Mequindox
Main ingredients and chemical name:: Mequindox, 2-acetyl-3-methylquinoxalinediium-1,4-diolate
【Structural formula】
Molecular Weight: 218.21
Chemical formula: C11H10N2O3
[Properties] This product is fresh yellow crystal or yellow powder, odorless, slightly bitter taste, and the color is gradually deep. This product is dissolved in acetone, chloroform and benzene, and slightly soluble in water, methanol, ether and petroleum ether.
[Pharmacological action] This product is a broad-spectrum antibacterial agent, and its antibacterial mechanism is to inhibit the deoxyribonucleic acid (DNA) synthesis of cells. It has a strong inhibitory effect on most bacteria, and it has a stronger effect on Gram-negative bacteria and is also effective against Treponema pallidum.
[Function and use] antibacterial drugs. It is mainly used for swine dysentery caused by Treponema pallidum, and also for enteritis caused by bacteria.
[Usage and Dosage] Oral: One dose, 1 kg body weight, 5-10 mg of cattle and pigs.
[Toxicity] This product has low toxicity, good tolerance to animals, and no carcinogenic or teratogenic effects.
[Adverse reactions] When the dose is higher than the clinical treatment amount by 3 to 5 times, or the application for a long time may cause adverse reactions or even death, the poultry is more sensitive.
[Note] It is for treatment only.
[content] ≥ 98.5%
[Packing] 25kg / cardboard drum.
[Storage] shading, sealed, kept in a dry and cool place
[Withdrawal period] 35 days.
[Validity period] 2 years.
[Approval No.] Veterinary Medicine (2011) 110504545
[Veterinary Drug Production License] (2011) Veterinary Drug Production Certificate No. 11050
【Manufacturer】
Company Name: Quzhou Weirong Pharmaceutical & Chemical Co., Ltd.



